
“We wanted to understand, how does this work? How are mitochondria capable of maintaining these electron inputs when oxygen is not the terminal electron acceptor?”

“This indicated that mitochondria could actually have partial function, even when oxygen is not the electron acceptor,” said Whitehead Institute postdoctoral researcher Jessica Spinelli. In the past, however, scientists have noticed that cells are able to maintain some functions of the electron transport chain, even in the absence of oxygen.
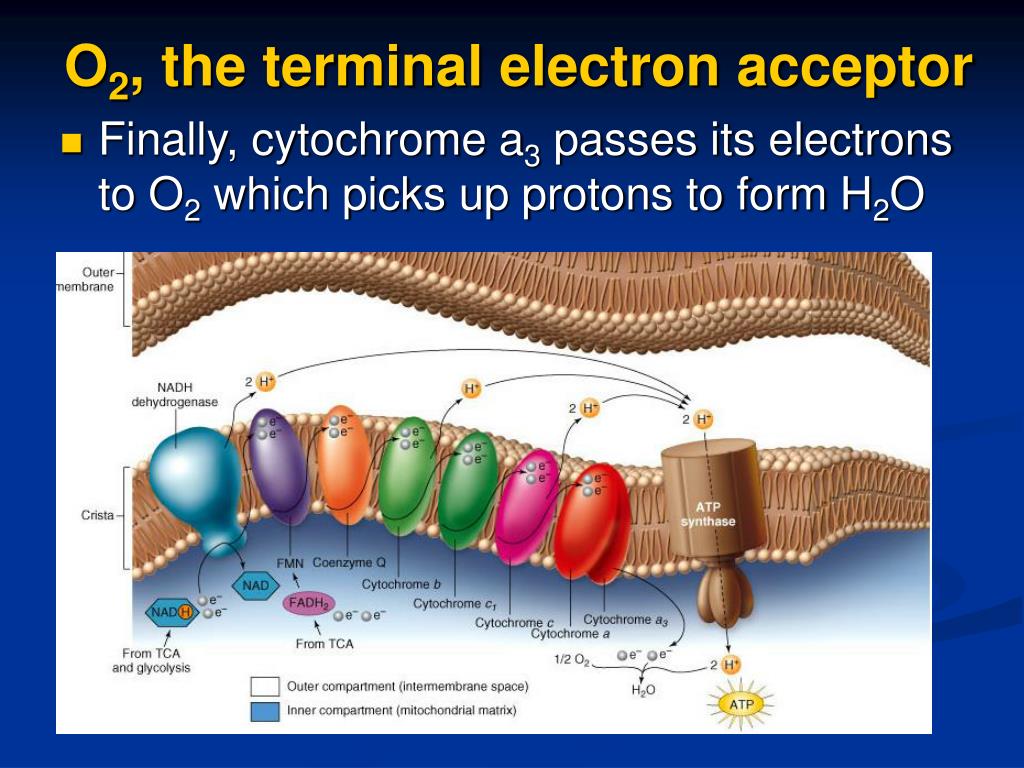
Oxygen serves as the terminal electron acceptor in series#
Through a series of reactions called the electron transport chain, electrons are passed along in a sort of cellular relay race, allowing the cell to create ATP, the molecule that gives our cells energy to complete their vital functions.Īt the end of this chain, two electrons remain, which are typically passed off to oxygen, the “terminal electron acceptor.” This completes the reaction and allows the process to continue with more electrons entering the electron transport chain. coli, although its spectral properties closely resemble those of cydABX-encoded cytochrome bd.Humans need oxygen molecules for a process called cellular respiration, which takes place in our cells’ mitochondria. Also, we present the novel data on the electrogenic function of appBCX-encoded cytochrome bd-II, a second bd-type oxidase that had been thought not to contribute to generation of a proton motive force in E. "WHAT'S NEW" IN THE REVISED ARTICLE: The revised article comprises additional information about subunit composition of cytochrome bd and its role in bacterial resistance to nitrosative and oxidative stresses. It has been proposed that the bo₃ oxidase can have two ubiquinone-binding sites with different affinities. coli membrane contains three types of quinones that all have an octaprenyl side chain (C₄₀). It also presents a discussion on the genetics and the prosthetic groups of cytochrome bo₃ and cytochrome bd. This review compares the effects of different inhibitors on the respiratory activities of cytochrome bo₃ and cytochrome bd in E. Terminal oxidases of aerobic respiratory chains of bacteria, which use O₂ as the final electron acceptor, can oxidize one of two alternative electron donors, either cytochrome c or quinol. A transfer of electrons from quinol to O₂ is served by two major oxidoreductases (oxidases), cytochrome bo₃ encoded by cyoABCDE and cytochrome bd encoded by cydABX. Under aerobic growth conditions, the terminal electron acceptor is molecular oxygen. Then electrons from reduced quinones (quinols) are transferred by terminal reductases to different electron acceptors. Substrate-specific dehydrogenases transfer reducing equivalents from various donor substrates (NADH, succinate, glycerophosphate, formate, hydrogen, pyruvate, and lactate) to a quinone pool (menaquinone, ubiquinone, and dimethylmenoquinone). In general, the bacterial respiratory chain is composed of dehydrogenases, a quinone pool, and reductases. Like most bacteria, Escherichia coli has a flexible and branched respiratory chain that enables the prokaryote to live under a variety of environmental conditions, from highly aerobic to completely anaerobic.


 0 kommentar(er)
0 kommentar(er)
